Unlock the potential of Asia with Zuellig Pharma's unique healthcare solutions.
Select Market
- Australia
- Brunei
- Cambodia
- China
- Hong Kong & Macau
- India
- Indonesia
- Japan
- Korea
- Malaysia
- Myanmar
- Philippines
- Singapore
- Taiwan
- Thailand
- Vietnam
Zuellig Pharma's depot in South Australia is a TGA-licensed facility that is approximately 1,300 sq.m. The facility has managed over 1,000 clinical trials since its foundation and currently manages more than 350 clinical trials.
Opportunities
- Clinical Trial Supplies Market to reach USD 5.7B by 2027.
- Healthcare CROs market in Australia and New Zealand valued at USD 1B in 2022.
- CAGR of 11.5% expected for healthcare CROs market from 2023-2030.
- Virtual clinical trials adoption to drive CRO industry growth in the region.
- Clinical trials in Australia offer R&D tax advantages and cost efficiency, driving industry growth.
Capabilities
- Global depot & cold chain logistics for reliable healthcare delivery.
- Sophisticated temperature-controlled. infrastructure adhering to industry standards (GDP, GMP, CSV, HSE).
- Smart Sourcing Solution for unparalleled access to medicine, incl. controlled drugs.
- Specialised cold chain for temperature-sensitive materials.
- Innovative patient-centric approach to clinical trials with depot-to-patient solutions.
Zuellig Pharma has a Brunei warehouse facility with an area of 7,330 sq.m. and a 225-pallet capacity for pharmaceutical, consumer, and medical device and diagnostics products. We serve over 90 healthcare customers nationwide, providing 100% coverage from government and private hospitals, specialist clinics, and chain pharmacies. Our certifications in Brunei for GDP, GDSP, and ISO 9001 (2015) ensure world-standard quality management system. Brunei's economic characteristics present immense business and tender opportunities, with a GDP of BND 5.8B as of Q3 2022 and 0.9% year-over-year growth, with a population size of approximately 450,000 as of 2022.
Opportunities
- Brunei's government tenders make up 70% of healthcare business.
- Thriving retail business with potential for chain pharmacy expansion.
- Growing oncology opportunities in public and private sectors.
- Potential for Brunei's Ministry to plan, promote, and develop medical tourism.
- Increasing demand for health and medical services due to ageing population.
Capabilities
- Leading healthcare provider in Brunei with 36 years of experience as only foreign invested company.
- Largest distributor and market leader, serving 9 of top 10 pharma clients in Brunei.
- Excellent RA capabilities, holding market authorisation for 25% of registered medicinal products with MOH.
- Innovative, automated warehouse system handles 80% of biologics and cold chain products in Brunei.
- Robust tender management team with excellent track record in handling MOH tenders at scale.
Zuellig Pharma's Cambodia facilities offer a newly-built distribution centre with an over 6,300 sq.m. warehouse and 1,200 sq.m. mezzanine for healthcare products, including 6,400 pallets in 6-high racking and 240 cold room storage pallets. With 90% coverage of ethical, pharmacy, and modern trade, we serve over 10,000 healthcare customers nationwide, including hospitals, clinics, and pharmacies, through 200 sales and marketing representatives. Our quality of work is recognised with certifications such as GDSP, GMP, GDPMDD, and ISO 9001:2015.
Opportunities
- Expansion of universal healthcare boosts medical services utilisation.
- NGOs continue to provide essential medicines.
- Basic medicines are vital due to regional production and low domestic manufacturing.
- Total pharmaceutical sales reached USD 386M in 2022 with per capita spending at USD 23.
- Pharmaceutical consumption rises to treat growing prevalence of chronic lifestyle diseases.
Capabilities
- Zuellig Pharma has 20 years' experience as healthcare provider in Cambodia, serving four top 10 Pharma clients.
- Largest cold-room facility in Cambodia with state-of-the-art infrastructure for healthcare.
- Proven cold chain tech maintains optimal quality during delivery.
- Passive packaging with unique thermal isolation chamber system.
- Patient-centric affordability and adherence programs.
- Custom mobile app for client program admin, enrolments, and updates.
Zuellig Pharma China's Beijing facility, with 20 years of clinical supply experience, is GDP/GMP compliant and a trusted partner for various clients in clinical trials, including pharmaceutical companies, CROs, CMOs, biopharmaceutical firms, medical device companies, and diagnostic firms.
Opportunities
- China's pharmaceutical market to reach USD 161.8B by 2023, accounting for 30% of global market.
- Ageing population and increased life expectancy driving rising demand for healthcare in China.
- Pharma sales in China projected to grow by 4.04% in 2022 and 3.58% in 2023.
- Chinese government promoting industry consolidation to regulate and control drug pricing.
- China imports biotechnology pharmaceuticals to meet growing domestic demand.
Capabilities
- Reliable global cold chain logistics network
- Sophisticated GDSP and GMP temperature-controlled facilities
- Smart sourcing solution for unparalleled medicine access
- Specialised cold chain solutions for sensitive materials in clinical storage
- Central distribution service for global studies
- Tailored repackaging services with multilingual labels
Zuellig Pharma has two warehouses in Hong Kong spanning over 48,000 sq.m., offering air-conditioned storage for 24,000 pallets and cold chain storage for 800 pallets. We serve 10,000+ healthcare customers, including hospitals, clinics, dentists, and drug stores. Zuellig Pharma moves 48,000 cubic meters of products annually and has won awards such as the HR Distinction Awards - Excellence in Employee Wellbeing and Occupational Health Award - Excellence Award of Joyful@Healthy Workplace Best Practices Award.
Opportunities
- Opportunities in China GBA for Hong Kong drugs and medical devices in public hospitals
- Predicted 3.5%-5.5% economic growth for Hong Kong in 2023
- High life expectancy in Hong Kong due to advanced healthcare and health consciousness
- Aging population driving demand for healthcare services and products
- Increased demand for consumer health post-COVID-19 due to raised health consciousness
- Smart hospitals and tele-health offer innovative and sustainable solutions for growing service demand.
Capabilities
- Over 40 years healthcare experience in Hong Kong
- Expert in healthcare solutions from storage to delivery
- Specialised in cold chain storage and distribution with certified facilities
- Over 20 years of vaccine and vaccination program experience
- Strong relationships with top healthcare companies in Hong Kong & Macau
- DoH approved and GMP certified secondary repackaging facilities.
Zuellig Pharma's facility in India is strategically-located in Mumbai to ensure that products and services are made available with the shortest leadtime. Covering over 1,530 sq.m., the facility offers one-stop solutions for every client's clinical trial needs, including a global depot and cold chain logistics network with temperature-controlled infrastructure and GDSP and GMP facilities for fast product and service availability. India is a cost-efficient and profitable market for major pharma, and biotech companies, as well as CROs, with local models meeting country standards.
Opportunities
- Clinical trial industry in India to grow at 8.2% per annum until 2030.
- Consistent patient population and easy access to modern medical technology in India.
- India offers a favorable currency exchange rate for pharma and biotech companies.
- Major pharma and biotech companies and CROs operate in India, incorporating local models to meet country standards, making it a cost-efficient and profitable market.
Capabilities
- Zuellig Pharma has a global depot and cold chain logistics network with advanced temperature-controlled facilities and GDSP and GMP facilities.
- Smart Sourcing Solution provides access to medicines and ancillaries, including controlled drugs.
- Specialised cold chain solutions available for temperature-sensitive materials.
- Strong Quality Management System (QMS) and operational excellence processes with a qualified team to offer CTSM services.
Zuellig Pharma provides complete coverage of ethical, pharmacy, and modern trade products in Indonesia, serving over 26,000 direct outlets with over 2,800 trained employees, 25 warehouses, 4 sales offices, and 8 APL partners' locations. The team has amassed numerous accolades, such as the HR Asia Best Companies to Work for in Asia and CNBC Indonesia Outstanding Company in Sustainability-Community Initiative awards.
Opportunities
- Indonesia launched its first digital health blueprint, aiming to expand inclusive healthcare coverage for its 270M population.
- The blueprint will help Indonesia accelerate its national goal of providing universal, affordable, equitable and quality care to all its citizens, using digital technologies.
Capabilities
- Zuellig Pharma offers integrated healthcare solutions in Indonesia, including distribution, commercialisation, patient care, data analytics, and importation.
- APL National Distribution Centre is the largest Pharma-grade warehouse in Indonesia, with 54,000 sq.m. space for pharma, consumer, and medical products.
- Over 13,000 orders are handled daily by 550 certified personnel, with 6,000 orders delivered via 200+ vehicles.
- The highest cold chain standards in Indonesia, with over 2,000 sq.m., 33 cold rooms, and 85 chillers.
Zuellig Pharma has an impressive partner depot facility in Japan, covering a total area of around 1.2 million sq.m., out of which 300,000 sq.m. are solely dedicated to pharmaceutical handling. Apart from its extensive size and specialisation, the partner depot also prioritises sustainability, evident through the installation of solar power generation systems and nighttime heat storage type air conditioning systems in all its offices.
Opportunities
- Japan's ¥10-trillion (USD 75B) national endowment fund signals growth in healthcare industry.
- Japan accounts for 4.7% of global clinical trial activity in 2022.
- Japan's developed healthcare system and ageing population increase demand for new treatments.
- Sakigake package accelerates drug and device approvals in Japan.
- Japanese Ministry of Health promotes innovation in pharmaceutical manufacturing.
Capabilities
- Partner depot in Japan obtained Green Management Certificate.
- Global logistics network with depots and cold chain infrastructure.
- Facilities comply with GDSP and GMP standards and offer advanced temperature control technology for cold chain solutions.
- Smart Sourcing Solution for exclusive access to medicines and controlled drugs.
- High standards of quality maintained, meeting GDP, GMP, CSV, and HSE requirements.
Zuellig Pharma's Korea facilities comprise 12 warehouses and a team of 150 sales and marketing representatives serving over 36,000 healthcare customers nationwide. We have received various accolades and certifications for sustainability, transparency in pharmaceutical distribution, and industry leadership.
Opportunities
- Strong healthcare system and popular medical tourism market.
- Booming digital health sector with patient-focused innovation.
- High purchasing power and one of the highest GDP per capita in APAC region.
- Rapidly expanding pharmaceutical industry with USD 17.9B reach and 9.6% CAGR in 2021.
- Thriving medical device industry with USD 7.9B reach and 10.6% CAGR in 2021, ranking 9th globally.
- Ageing population with growing healthcare demands.
- Increasingly recognised as a key destination for clinical trials in the Asian pharmaceutical market.
Capabilities
- 25 years experience as a healthcare service provider in South Korea.
- Operate South Korea’s largest cold-room facility.
- South Korea’s largest specialty and vaccine distributor, and Asia’s clinical logistics leader.
- Preferred go-to commercialisation partner for high compliance standards and transparency.
- Customised technology solutions for eCommerce, block-chain, and business analytics.
Zuellig Pharma's Malaysia facilities include a main warehouse in Bukit Jelutong and an approximately 7,000 sq.m. warehouse for dialysis solutions, medical supplies, and surgical lenses. With two branch offices and a branch warehouse, Zuellig Pharma has 100% coverage of hospitals, clinics, and pharmacies with over 15,000 customers across Malaysia. We have won numerous awards for quality and certifications.
Opportunities
- Quality healthcare system, top global medical tourism market.
- Growing patient-centric digital health innovation in Malaysia.
- High allocation from Budget 2023 for healthcare.
- Medical device industry to reach USD 2.2B in 2023.
- Ageing population driving increasing demand for healthcare services.
Capabilities
- 84 years experience as healthcare provider in Malaysia.
- Largest cold chain facility, eZCooler as an advanced passive packaging solution.
- End-to-end integrated solutions for entire healthcare value chain.
- Actively drive sustainability and partner with clients on special projects to meet carbon savings objectives.
Zuellig Pharma's Myanmar facilities provide comprehensive coverage of ethical, pharmacy, and modern trade services with over 90% reach. Our facilities are certified in GDSP, GMP, and ISO, ensuring top-quality cold chain facilities, passive packaging, and patient-centric programs for affordability and adherence. With over 4,000 healthcare customers nationwide and a team of 90 highly-trained sales representatives, we offer exceptional service.
- National health insurance system reduces healthcare expenses and improves accessibility.
- Companies Act encourages foreign investment in the pharmaceutical sector.
- National efforts combat malnutrition, undernutrition, and obesity through health and educational programs.
- International aid packages boost local health facilities in combating COVID-19.
- Zuellig Pharma has 8 years of experience in Myanmar, serving 6 out of the top 10 pharma clients.
- Our top-notch cold-room facilities use advanced infrastructure.
- Our cold chain transport tech and packaging use thermal isolation chambers for quality maintenance.
- We provide patient-centric solutions for affordability and adherence.
- Custom mobile apps are available for client program administration and real-time updates.
Zuellig Pharma in the Philippines has seven distribution warehouses covering over 59,000 sq.m. of distribution centre space for pharma, consumer, medical devices, and diagnostics products. We have over 1,700 employees nationwide and have distributed to over 19,000 physicians, 700 private hospitals, 7,000 chain drug stores, and more. We have been recognised for our quality of work and sustainability efforts.
Opportunities
- Ophthalmology agency business shows consistent growth building on B&L.
- Diagnostic and consumable business is rapidly growing, currently 53% SOB in MDI.
- Animal health business is rapidly growing, especially in the pet segment.
- Capitalising on client relationships to drive commercialisation initiatives.
- Potential to improve awareness and utilisation of value-added services (VAS) for higher operating revenue and client conversion.
Capabilities
- Over 100 years of experience in pharma distribution in the Philippines.
- 100% coverage of hospitals, pharma, modern trade accounts & 50k+ customers nationwide.
- Strong market presence in 86 therapeutic classes.
- Wide range of services incl. Repackaging, Samples Distribution, Product Destruction & Trade Marketing (CHC).
- Innovative tech solutions incl. eZHealth, eZRx & e-Commerce solutions.
- Patient-centric programs for adherence, access & affordability.
Zuellig Pharma's Singapore facilities cover over 44,000 sq.m. across three warehouses and serve over 6,000 healthcare customers with extensive ethical, pharmacy, and modern trade coverage. We have received various accolades and certifications, such as the HR Asia Best Companies to Work (2020) for Singapore, IDC Future Enterprise Awards (2022), and National Awards (COVID-19) 2022 recipient for President's Certificate of Commendation for Singapore.
Opportunities
- Top destination for medical tourism with excellent healthcare.
- Singapore driving patient-centred innovations in digital health.
- High GDP per capita reflects strong purchasing power in APAC.
- Medical device industry projected to reach USD 3.5B by 2022.
- Increasing demand for health services due to ageing population.
Capabilities
- Zuellig Pharma delivers 85+ years of healthcare excellence in Singapore.
- Largest cold-room facility with innovative infrastructure.
- Top secondary repackager with serialisation capability.
- Patient-centric programmes for affordability and adherence, with custom mobile app.
- Tailored technology solutions for eCommerce, blockchain and analytics.
Zuellig Pharma in Taiwan boasts five warehouses spanning over 65, 000 sq.m. and catering to pharmaceuticals, consumer goods, and medical devices. We offer 100% coverage of ethical, pharmacy, and modern trade services, serving 20,000+ healthcare customers with over 200 sales and marketing representatives. We have received accolades like the National Healthcare Quality Award and National Biotechnology and Medicine Care Quality Award.
Opportunities
- National health insurance budget growing at 2.9% - 4.5% annual rate.
- Strong digitalisation trend in health sector, particularly in telemedicine and AI diagnosis.
- Mature environment for clinical trials phases 1-3.
- Government-funded local biotech companies researching and manufacturing.
- Increasing healthcare demands due to ageing population and low fertility rate.
Capabilities
- Over 35 years of healthcare experience in Taiwan.
- Strong relationships with 90+ multinational healthcare companies.
- Taiwan's only ultra-low cold chain system and largest cold chain storage.
- Patient care team of 50 nurses serving 60,000+ patients.
- Customised eZ-solution app for program administration and real-time updates.
Zuellig Pharma runs four warehouses in Thailand, with 56,400 pallet storage space and a full range of temperature-controlled cold chain rooms for various products. We offer 98% customer coverage of ethical, pharmacy, and OTC, serving over 22,000 healthcare customers with 500 sales and marketing representatives, and have distributed 87% of vaccines to date. We have received numerous accolades, including nine awards at the 2022 TCCTA Contact Center Awards.
Opportunities
- Rising demand for healthcare due to increased focus on personal health and COVID-19.
- Ageing population in Thailand leads to increased treatment for chronic diseases.
- Universal Health Coverage scheme provides broad coverage, with return of foreign patients.
Capabilities
- Zuellig Pharma has 75+ years of healthcare experience in Thailand.
- Largest cold-chain facility for healthcare products with various temperature protocols.
- Fleet of air-conditioned transportation vans with innovative eZCooler packaging.
- One-stop-service for redressing under GMP.
Zuellig Pharma's Vietnam facilities comprise five warehouses with over 23,000 AC pallets and 1,700 CC pallets, catering to various healthcare products. We provide 100% coverage across all medical channels, serving over 60,000 healthcare customers. Our certifications include GDP, GCP, GMP, GSP and GSDP, GDPMD, ISMS, and ISO.
Opportunities
- Ageing population drives long-term demand for prescription drugs.
- Growing middle class leads to increasing demand for quality healthcare services.
- Significant contribution from imported pharmaceuticals, APIs, and medical devices.
- Abundance of M&A opportunities across healthcare verticals.
Capabilities
- Over 30 years healthcare service experience in Vietnam.
- Facilities in Vietnam meet FM Global Platinum standards and use efficient energy features.
- Patient-centric programs for affordability and adherence.
- 100% direct tender penetration and 99% sales coverage through call plans for retail channels.
- Comprehensive ordering platforms and technology consulting services.
Solutions
At Zuellig Pharma, we bridge the gap between clients and customers in the healthcare value chain by providing customised and innovative healthcare solutions that ensure unbeatable access to care throughout Asia.
- Distribution
- ZP Therapeutics
- Clinical Reach
- Technology Solutions
- Patient Solutions

State-of-the-art end-to-end distribution solutions across 13 markets
Distribution
Experience unparalleled access to the healthcare market with Zuellig Pharma. Our comprehensive range of pharmaceutical distribution services and tailored healthcare solutions empower your business to reach its full potential. As a trusted leader in the industry, Zuellig Pharma delivers custom healthcare solutions that meet your unique needs.
Discover More
Understands consumers, customers and market in Asia
ZP Therapeutics
Zuellig Pharma is your trusted commercialisation partner in the healthcare industry. We specialize in delivering innovative pharma solutions to enhance patient access to care. Our flexible partnership models cater to diverse client needs. With extensive expertise in Asia, we provide regulatory and governmental affairs support, along with sales and marketing strategies to help businesses expand their reach and impact.
Discover More
The future of clinical trials is brighter than ever.
Clinical Reach
Partner with Zuellig Pharma for seamless management of clinical trial supply chains. From regional studies to complex global multi-center trials, our expertise spans diverse scales. With our global product sourcing, integrated storage and pharmaceutical distribution solutions, and localized returns management, we ensure compliance with local and international healthcare regulations for your clinical needs.
Discover More
Your digital platform of choice
Technology Solutions
Revolutionise Asia's healthcare ecosystem with Zuellig Pharma's innovative pharmaceutical software platforms. Our advanced solutions bridge the gaps, enhancing access and outcomes for clients, healthcare professionals, and patients. With cutting-edge technology, businesses can streamline operations, expand their reach, and deliver the care patients deserve.
Discover More
Personalised and innovative healthcare support
Patient Solutions
Experience patient-centric care with Zuellig Pharma's Patient Solutions platform. Our personalised healthcare solutions and support empower patients to take control of their well-being. With innovative features like mobile apps, home care services, and disease management programs, we enhance the patient experience and improve health outcomes.
Discover MoreSegments
Long and lasting relationships with leading healthcare pharmaceutical companies across multiple industry verticals.

Technology Solutions
Digital technologies are at the core of our services. Learn how governments and partner companies are driving efficiency and effectiveness with Zuellig Pharma.
eZTracker
eZTracker by Zuellig Pharma is an advanced supply chain management solution that helps businesses track and monitor their healthcare products in real-time.
eZRx
eZRx by Zuellig Pharma is a cutting-edge platform that streamlines pharmaceutical ordering and delivery processes. eZRx enables seamless communication between healthcare professionals and suppliers for efficient & effective medication management.

About Us
We provide world-class distribution, technology, commercial, and integrated health solutions and services to support the growing healthcare needs in this region. The company was started a hundred years ago and has grown to become a multi-billion dollar business covering 16 markets with over 12,000 employees.

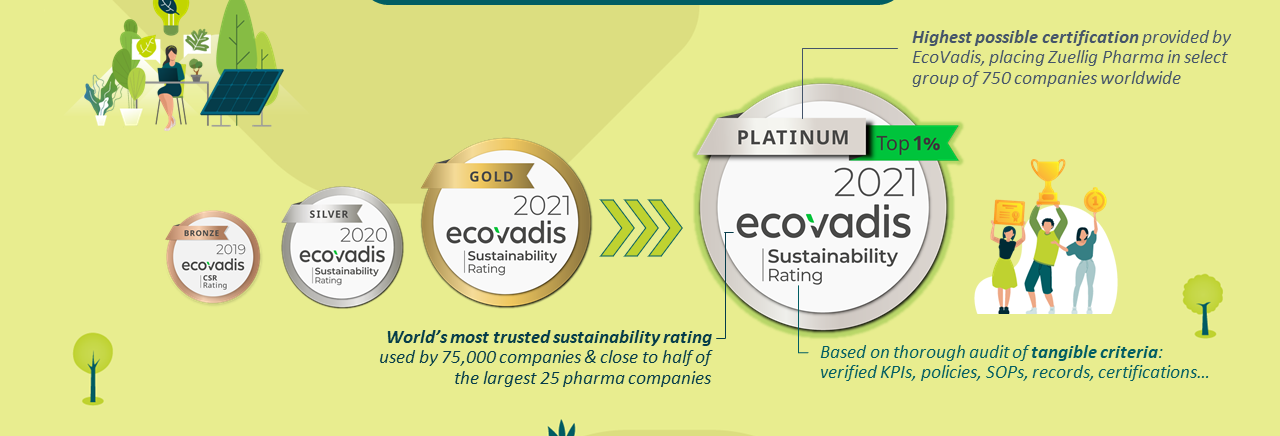
Sustainability
At Zuellig Pharma, we prioritise sustainability for a healthier future through ethical business practices, resource conservation, and community support. Our EcoVadis Platinum Rating for 2023 showcases our dedication to pursuing a better tomorrow for our generation and beyond.
News and Insights
Discover what Zuellig Pharma is doing across the globe.